Local Initiatives
Choosing Wisely Manitoba is leading many quality improvement initiatives to introduce evidence-based changes and improvements to Manitoba’s health care system. Our goal is to improve appropriateness of care, patient and provider experience, health system efficiencies and sustainability by working with our healthcare stakeholders to build capacity for research stewardship and quality improvements projects that aim to reduce unnecessary tests, treatments and procedures in Manitoba.
How are we implementing Choosing Wisely Manitoba?
- Thyroid Function Testing
- Immune Globulin
- Improving the Appropriate Use of MRI for Lower Back Pain
- Red Blood Cell Utilization
- Improving Preoperative Diagnostic Testing
- Reducing Unnecessary Prostate-Specific Antigen (PSA) Testing
- Discontinuation of Creatine Kinase for diagnosis of Acute Coronary Syndrome
- Activated Partial Thromboplastic Time
- ESR and CRP Testing
- Just One
- Tissues for Disposal
- Appropriate Vitamin D Testing
- Appropriate Use of FOBT
Thyroid Function Testing
The Shared Health laboratory, Section of Endocrinology and Metabolism and Pediatric Endocrinology have collaborated to develop a clinical guideline to streamline thyroid function testing in primary care; effectively automating the reflexing of free T4 and free T3 tests as appropriate within the testing process. The provincial clinical guideline will be supported by revised laboratory requisitions to reflect the reflex testing model and will include physician education and decision support embedded into requests. Dynacare laboratories are also being engaged to adopt similar policies.
Both Choosing Wisely Canada and Choosing Wisely USA have recommendations surrounding the testing of Free T4 and Free T3.
Canadian Society of Endocrinology and Metabolism – Don’t use Free T4 or T3 to screen for hypothyroidism or to monitor and adjust levothyroxine (T4) dose in patients with known primary hypothyroidism. T4 is converted into T3 at the cellular level in virtually all organs. Intracellular T3 levels regulate pituitary secretion and blood levels of TSH, as well as the effects of thyroid hormone in multiple organs. Therefore, in most people a normal TSH indicates either normal endogenous thyroid function or an adequate T4 replacement dose. TSH only becomes unreliable in patients with suspected or known pituitary or hypothalamic disease when TSH cannot respond physiologically to altered levels of T4 or T3.
American Society for Clinical Pathology – Don’t order multiple tests in the initial evaluation of a patient with suspected thyroid disease. Order thyroid-stimulating hormone (TSH), and if abnormal, follow up with additional evaluation or treatment depending on the findings. The TSH test can detect subclinical thyroid disease in patients without symptoms of thyroid dysfunction. A TSH value within the reference interval excludes the majority of cases of primary overt thyroid disease. If the TSH is abnormal, confirm the diagnosis with free thyroxine (T4).
Improving the Appropriate Use of Immune Globulin
Manitoba was one of the highest per capita users of immune globulin products in Canada (40% over that of Ontario and the Atlantic provinces). As a result of COVID-19, Canadian Blood Services anticipates shortages in the coming year resulting from reduced plasma collections. This Targeted Practice Improvement aligns Manitoba with practices in other jurisdictions and implements recommendations from the Prairie Collaborative Immune Globulin Utilization Management Framework Project.
In December 2020, Best Blood Manitoba and Shared Health Transfusion Medicine Labs implemented a new immune globulin approval process in Manitoba. The new process is based on recommendations from the Prairie Collaborative Immune Globulin Utilization Management Framework Project, which was an initiative to review evidence of immune globulin therapy benefits and appropriate dosing from a number of sub-specialties. Clinical specialists from across Manitoba, Alberta and Saskatchewan collaborated to establish and ratify this framework in 2018.
These specialty areas include Dermatology, Hematology, Immunology, Infectious Diseases, Transplant Medicine, Neurology and Rheumatology.
This project will help maintain availability and continued access for patients that meet criteria for use in Manitoba, ensure patient therapy is monitored and dosed appropriately, reduce potential risk and harms and foster stewardship amongst all users of blood products in Manitoba.
More information and provider resources can be found on the Best Blood Manitoba website.
Improving the Appropriate Use of MRI for Lower Back Pain
Lower back pain is very common and 50-90% of people will experience lower back pain at some time in their lives. It is also a common reason for visits to primary care. In the absence of red-flag symptoms, MRI is not recommended for lower back pain as it rarely aids in patient treatment or outcomes.
A pilot project at Boundary Trails Health Centre saw a 56% decrease in ordering of these tests after physician and patient education materials were developed and implemented at the two large clinics surrounding Boundary Trails. By decreasing unnecessary MRI testing for low back pain, Manitoba will see reduced wait times for patients who require higher value MRI imaging.
In 2020, approval was granted for expanding these guidelines provincially.

Red Blood Cell Utilization
Background Information:
Manitoba was one of the highest users of red blood cells per capita in Canada. While each unit transfused has benefits; it also carries inherent risks to the patient and uses valuable laboratory and nursing resources and in some cases provides no health benefit to the patient. The Shared Health Transfusion Medicine Program, Best Blood Manitoba and Choosing Wisely Manitoba have teamed up to use evidence-based practice in order to optimize laboratory and clinical transfusion practices and improve the appropriateness of each unit transfused. We will be providing 24/7 transfusion on call resources to consult on complex cases.
The New Guidelines:
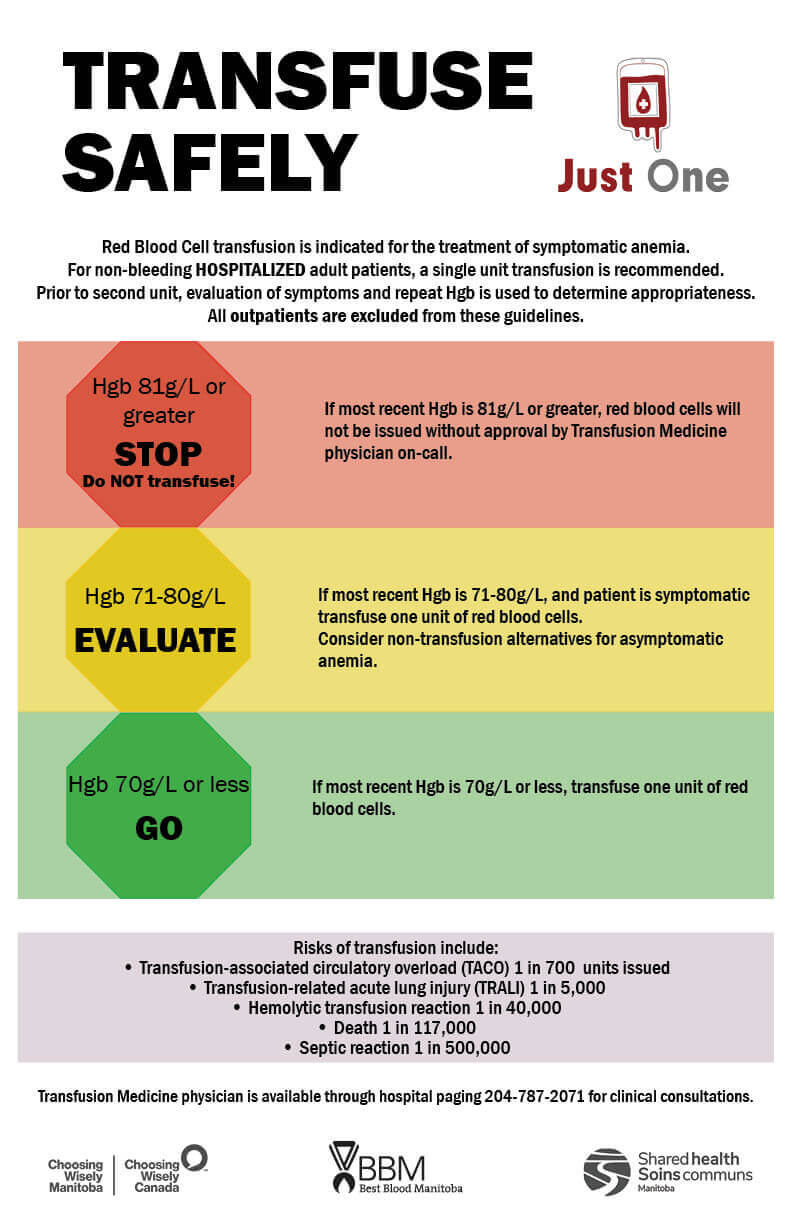
Red blood cell transfusion is indicated for the treatment of symptomatic anemia. For symptomatic non-bleeding adult patients, a single unit transfusion is recommended. If a second unit is required; evaluation of symptoms and hemoglobin should be used to guide appropriateness. There is also ample evidence that transfusions in patients with higher hemoglobin ranges may not benefit as much from transfusion as those under 70 g/L. Thresholds have been put in place to transfuse at higher levels.
The changes only apply only to stable, non-bleeding in-patients and will not include transfusions in the following settings: paediatrics, CancerCare, Emergency departments, intra-op and actively bleeding patients.
Phased Process Implementation:
The process change was rolled out in phases, beginning with Southern Health-Santé Sud in January 2020 and Prairie Mountain Health in early March 2020. Due to COVID-19 and concern over potential blood shortages in Canada during the pandemic, we were able to quickly implement the rest of Manitoba’s health regions at the end of March 2020.
Project Poster:
For more information on this project, visit the Best Blood Manitoba website here.
Improving Preoperative Diagnostic Testing
In Manitoba, many patients presenting for low-risk surgery were receiving full preoperative assessments despite the fact that these assessments did not improve outcomes. In 2014 a multidisciplinary team was formed to restructure the way preoperative assessment is carried out in Manitoba. The goal was to ensure that all Manitobans receive the necessary and appropriate preoperative diagnostic testing for minor surgeries through the sustainable implementation of a standardized, evidence-informed clinical practice guideline.
This project started with an examination of the barriers and facilitators to guideline adoption and implementation including further analysis of audit results, stakeholder consultations and a literature review. The results of this work were then used to develop and implement a new Routine Preoperative Lab Test Guideline. A series of interventions were then developed aiming first to reduce unnecessary testing in cataract surgery and then to reduce unnecessary testing in other types of surgery.
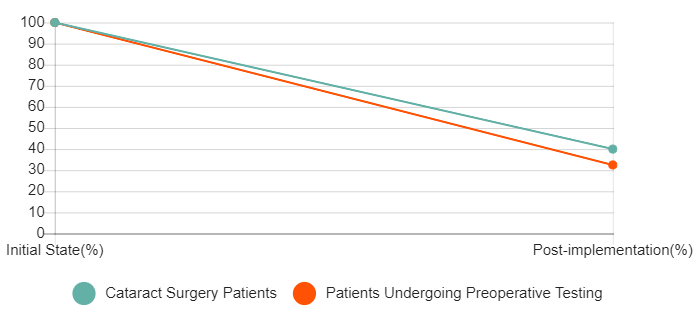
The implementation of a revised Cataract Surgery History and Physical (H&P) Form emphasized that tests are not usually required for cataract surgery and eliminated cuing for unnecessary preoperative tests. At the same time a Red-Green project eliminated preoperative History and Physicals for ~60% of cataract surgery patients. Pre and post-implementation audits showed a 77.5% reduction in patients undergoing preoperative testing from pre to post-implementation.
Building on what we learned through the cataract project, on July 11, 2016, five revised documents were implemented to help reduce cues that prompt unnecessary testing for all other types of surgery. The revised documents were implemented in eight surgical sites across Winnipeg.
Four Documents:
- Revised Routine Preoperative Lab Tests Guidelines
- WRHA Preoperative History & Physical Form
- Patient Quick Reference Cover Letter
- Preoperative Care Cover Letter
Our primary outcome, to reduce preoperative diagnostic testing by 25%, was evaluated through retrospective chart reviews of two random samples of surgical patients during typical weeks in the WRHA. The results demonstrated a significant (p<0.0001) 35% reduction in preoperative diagnostic testing.
In order to review adherence to the guidelines and to support implementation by providing feedback and supporting materials, we have conducted approximately 10 audits of surgeons in each of the 8 surgical sites in Winnipeg (excluding cataract and pediatrics). The results revealed that the majority of surgeries were compliant with the guidelines (60%) but that there is a large range in the number of unnecessary tests ordered between surgical specialties from 24% of tests ordered are unnecessary to 72% are unnecessary. Based on these results our next step is to provide feedback to individual surgeons including supporting materials to increase adherence to the guidelines.
See a Summary of the Evaluation Results
For a copy of the full evaluation please contact us at [email protected].
Reducing Unnecessary Prostate-Specific Antigen (PSA) Testing
The PSA blood test is a test for prostate cancer, the most common cancer among men in Canada. The test has been used widely for early detection of the disease despite the fact that it does not significantly decrease overall prostate cancer mortality. Further, PSA testing can lead to a high number of false-positives, leading to over diagnosis, over treatment, and a potential decrease in quality of life. PSA levels may be elevated for many reasons and most men with high PSAs don’t have prostate cancer. Usually when prostate cancer is detected these cancers grow slowly and have a good prognosis, even without treatment.
Many provinces in Canada along with the Canadian Urology Association, Canadian Cancer Society, Canadian Task Force on Preventive Health Care and Prostate Cancer Canada have recommendations around when men should have a PSA test to screen for prostate cancer. Most recommendations limit testing to within certain age parameters and risk factors.
An initial review of the current state of PSA testing in Manitoba revealed that every year thousands of tests are carried out on men who are not at high risk of life threatening prostate cancer. Choosing Wisely Manitoba is working with key stakeholders to examine current guidelines that reinforce appropriate testing parameters and clinical indications for PSA testing in Manitoba. In addition we are studying ways to educate patients and support physicians in engaging in healthy conversations about PSA testing.
Discontinuation of the use of creatine kinase (CK) for the diagnosis of acute coronary syndrome (ACS)
Background Information:
Creatine kinase (CK) has been a traditional biomarker for identifying patients with acute myocardial infarction (AMI). High-sensitivity cardiac troponin (hsTnT), however, has been shown to be significantly more sensitive and specific in identifying myocardial ischemia and has become the preferred biomarker for myocardial necrosis among consensus recommendations.1 For example the 2018 American Heart Association consensus guidelines on the universal definition of myocardial infarction recommend high sensitivity troponin assays for the diagnosis of ACS due to their superior sensitivity and specificity for myocardial injury; other biomarkers, such as CK, are not recommended.7
This has called into question the clinical utility and cost effectiveness of CK in the work-up of chest pain in the emergency department (ED).2-5
A study conducted by researchers in the University of Manitoba Departments of Internal Medicine, Section of Cardiology and Emergency Medicine6, aimed to clarify whether CK provided any added diagnostic value over hsTnT alone. A retrospective review of all adult patients (36,251) presenting to St. Boniface Hospital Emergency Department in 2017 was conducted. Patients presenting with cardiac complaints who had non-diagnostic hsTnT and positive CK were identified, and their charts reviewed to determine whether a diagnosis of AMI was made, and whether they experienced subsequent cardiovascular morbidity or mortality. The results revealed that CK provided no diagnostic benefit over hsTnT alone in >99.9% of cases.
Based on the results of this, and other studies3,5 that demonstrate that routine CK testing does not provide a significant benefit to patient care and therefore represents an unnecessary system cost, the goal of this resource stewardship project will be to eliminate routine CK testing for the diagnosis of AMI in emergency departments across the WRHA.
In July 2020, the project was rolled out across Manitoba.
Clinical Practice Change:
Discontinuation of the use of creatine kinase for the diagnosis of acute coronary syndrome (ACS) Clinical Practice Change
References:
- Jaffe AS. New standard for the diagnosis of acute myocardial infarction. Cordial Rev 2001; 9(6): 318-22.
- Volz KA, Horowitz GL, McGillicuddy DC, Grossman SA, Sanchez LD.Should creatine kinase-MB index be eliminated in patients with indeterminate troponins in the ED? Am J Emerg Med 2012; 30(8): 1574-6.
- Volz KA, McGillicuddy DC,Horowitz GL, Sanchez LD. Creatine kinase-MB does not add additional benefit to a negative troponin in the evaluation of chest pain. Am J Emerg Med 2012; 30(1): 188-90.
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008; 118(21): 2200-6.
- Le RD, Kosowsky JM,Landman AB,Bixho I, Melanson SE, Tanasijevic MJ. Clinical and financial impact of removing creatine kinase-MB from the routine testing menu in the emergency setting.Am J Emerg Med 2015; 33(1):72-5.
- Wiens EJ, Arbour J, Thompson K, Seifer C. Routine creatine kinase testing does not provide clinical utility in the emergency department for diagnosis of acute coronary syndromes. BMC Emerg Med. 2019;19:37. doi: 10.1186/s12873-019-0251-4.
- Thygesen K, Alpert J, Jaffe A, Chaitman B, Bax J, Morrow D, White H: the Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e000–e000.
Project Poster:

Activated Partial Thromboplastic Time
The suboptimal utilization of aPTT testing has been identified as a prevalent problem across all health regions in Manitoba. As part of Choosing Wisely Manitoba and as per the aPTT testing recommendations issued by Choosing Wisely Canada, Shared Health’s Hematology discipline provided direction and new guidelines for the ordering of aPTT testing. Following this clinical practice change in October 2015 aPTT test requests have decreased provincially by 82%.
$20,000
Estimated reduction in aPTT test requests per month
$57,000
Anticipated savings in supply costs
Related clinical practice changes can be found here:
ESR and CRP Testing
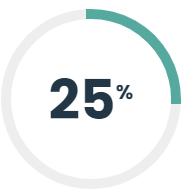
C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR) testing generally offer the same information on a patient, looking for evidence of inflammation in the body. Often, physicians order both of these at the same time, however they both provide relatively the same information except in special circumstances. In collaboration with Infectious Disease specialists and Rheumatologists, Shared Health Laboratory has established that we can safely not provide both tests to general practitioners unless they have the approval of a Hematologist or Hematopathologist. This has resulted in a 25% decrease in combined testing. In addition, since this test is not needed as a stat test, the project was able to consolidate the number of sites where this test is performed, adding to province-wide efficiencies.
Observed decrease in combined testing of C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR)
Related clinical practice changes can be found here:
Just One is an awareness campaign intended to educate and remind clinicians of best practices and guidelines around red blood cell infusions and their -potential harms.*
A clinical practice guideline was distributed to all Manitoba clinicians in the late 2018 to educate clinicians on appropriateness criteria for blood transfusions in stable, non-bleeding patients. The key messages were
- Just one unit of blood should be ordered for stable patients with no clinical bleeding.
- Re-assess the patient before requesting each unit of blood. Indications for a receiving additional units are:
- Active blood loss
- Ongoing symptoms of anemia
These are evidence-based recommendations made by the Canadian Society for Transfusion Medicine and Choosing Wisely Canada.
The materials included possible Patient impacts and system improvements including:
Patient Impact:
Each unit of blood administered has the potential to cause adverse reactions. These can range from as minor as a rash or fever to more severe reactions such as acute respiratory distress or even death. The reactions to a transfusion may happen immediately or it may be delayed as the body responds to the donors red cells via the patient’s immune system. In addition, every unit of blood transfused may increase the likelihood of the patient developing antibodies to donor red cells resulting in challenges in finding compatible units if/when the patient requires further transfusions.
System Improvements:
Reduction in the number of transfused red cells can reduce the significant pressure on Canada’s blood supply, ensuring availability for patients who require lifesaving units. System improvements can also be seen by reducing workload on Transfusion Medicine departments and Canadian Blood Services to collect, process, distribute and issue these products.
Choosing Wisely Manitoba, in partnership with Shared Health, has developed posters and postcards that relay safe transfusion practice regarding single unit transfusion orders. The postcard will be attached to each red cell unit as it is issued from the blood bank.
Posters and postcards were distributed across the province to hospital areas that commonly transfuse red blood cells. Postcards were attached to the units of blood for approximately one month whenever more than one unit was ordered.
*(May not apply to outpatient transfusions for oncology patients.)
Tissues for Disposal
Human tissues removed during a biopsy or surgical procedure are typically sent to a pathology laboratory for diagnostic examination and testing. Under the framework of Choosing Wisely Manitoba, and with the support of Orthopedic Surgeon and Clinical Champion, Dr. Eric Bohm, Shared Health conducted a retrospective review of approximately 300 orthopedic surgery cases (representing one surgeon’s annual caseload) to correlate pathology findings with the original diagnostic imaging report. As evidence and experts had suggested, the results of the review supported the case that pathology testing on tissues removed from the body during orthopedic procedures do not provide additional clinical data making the original diagnostic imaging report clinically sufficient. With no value added to patient care, these tissues are now recommended for exemption from requiring pathological review and instead recommended for immediate disposal.
New standard operating procedures to eliminate testing from elective hip and knee replacement surgeries were developed and implemented at Concordia Hospital, Grace Hospital and Boundary Trails Health Centre. Now fully implemented at all provincial orthopedic surgery sites, the disposal of these tissues is freeing pathologists’ for other important work.
On average pathology testing was reduced from 204 tests per month to 13 tests per month (April – December, 2017). That represented a 93% reduction in pathology testing following the implementation of the new standard operating procedures. Collaboration with surgeons has led to the development of processes to facilitate immediate disposal and improve workflow and efficiencies.
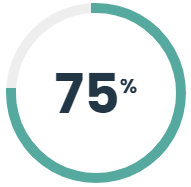
Reduction in pathology testing following the implementation of the new standard operating procedures.
Related clinical practice changes can be found here:
Appropriate Vitamin D Testing
Vitamin D (25(OH)D) deficiency testing in Manitoba has increased exponentially over the last eight years, from approximately 5,000 in 2006 to 50,000 completed tests in 2015/16. Evidence based guidelines advise against routine testing.
CWMB implemented new ordering criteria and a new requisition (supported by Choosing Wisely Canada and Manitoba Endocrinologists) in early 2016. Since project implementation, there has been a 90% reduction in this testing, which correlates to efficient use of resources and better care. Manitoba has improved service delivery for medically indicated and priority tests by implementing this change, as well as making way for a redirection of resources to other diagnostic areas of need.
$800,000
Investment that could be redirected to other critical diagnostic areas
Related clinical practice changes can be found here:
Appropriate Use of FOBT
In the absence of a national Choosing Wisely recommendation on Fecal Occult Blood Testing (FOBT), CWMB has assumed a leadership role in engaging Shared Health Laboratories and Gastroenterology specialists to conduct research and present the evidence on appropriate use for FOBT in our province. The guaiac FOBT was designed to detect hidden blood in stool, making it an effective screening tool for colorectal cancer screening. However, the test is also commonly used (off-label use) in hospitalized patients to detect gastrointestinal bleeding in the investigation of anemia. A practice change was issued to support the recommendation that use of the guaiac FOBT should be restricted to the approved indication of screening for colorectal cancer in asymptomatic patients and should not be performed on hospitalized patients for investigation of anemia. CWMB has adopted this as its first “made in Manitoba” recommendation and hopes to lead other provinces in moving toward enforcing the appropriate use of this test. Post project analysis shows a 74% decrease in this testing on inpatients.
Related clinical practice changes can be found here:
